T-Shirt Sends ECG Signals To Your Smartphone
By Joel Lindsey
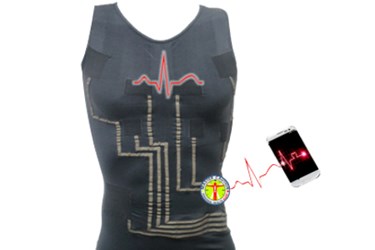
HealthWatch, a medical device company based in Tel Aviv, Israel, demonstrated its hWear line of wearable ECG-sensing garments this week at the American Telemedicine Association (ATA) Annual Meeting.
The hWear line of wearable medical devices is designed to provide high-quality ECG monitoring for its wearers. The company describes the line of products as “revolutionary digital, heart-sensing [garments] incorporating interwoven textile electrodes for continuous monitoring of hospital quality, 15-lead ECGs. . . . hWear t-shirt garments measure the highest quality vital signs all without adhesives, gels, or shaving preparations for both men and women. The garments are machine washable and compatible with most cardiac telemetry systems.”
HealthWatch’s ECG-monitoring shirts have been designed to work in tandem with the company’s MasterCaution line of software products, which collect and report measurements back to wearers on their mobile devices. MasterCaution software has also been designed to provide cloud-based communications to enable more effective monitoring and reporting of cardiac health.
“We are restoring the ‘wear’ into wearable technology,” said Uri Amir, HealthWatch CEO. “Unlike other products that report only heart rate, our new healthwear garment is a true medical device monitoring full 15-lead ECGs along with other physiological vital signals. It will change the future of personal monitoring offering around-the-clock peace of mind to users — wherever their lifestyle takes them.”
The line of hWear ECG-monitoring shirts has received both CE and FDA approval, though the MasterCaution software has yet to receive FDA clearance.
“The eHealth, TeleHealth, mHealth, and tele-cardiology industries can now deploy intensive-care quality telemonitoring without affecting the lifestyle of their users ranging from patients within a hospital environment, to homecare settings, to heart attack survivors, to the active elderly seeking better coverage of their health, or to users wishing to have direct contact with their expert personal physician from anywhere,” Amir said in a press release. “We will be submitting the MasterCaution telemonitoring device for FDA clearance in a couple of months.”
According to an article on MobiHealthNews, HealthWatch will initially market the garments to hospitals, for patients who need longer-term monitoring than a Holter monitor can provide. After establishing the devices’ efficacy in a clinical setting, the company plans to launch “a wellness-focused, direct-to-consumer product. The company is also interested in the telemonitoring space, where the tshirt could automatically alert doctors if it detected an arrhythmia or a heart attack.”
Wearable medical devices represent a popular and growing field in 2014. In addition to HealthWatch’s recent announcement, Apple continues to make headlines by hiring a growing number of well-known biomedical device experts. Most recently, an article published by Med Device Online highlights the company’s newest personnel acquisitions as it continues developing its much-touted iWatch and biometric EarPod devices.
Image credit: HealthWatch Technologies
