Qorvo Biotechnologies Omnia Instrument Testing System
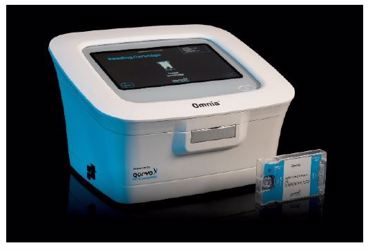
This white paper describes the Qorvo Biotechnologies Omnia testing system using bulk acoustic wave (BAW) sensor technology and performance data generated in a recent study conducted by the NIH as part of the RADx initiative for SARS-CoV-2 antigen testing. The Qorvo Omnia SARS-CoV-2 Antigen test was granted EUA authorization for the qualitative detection of SARS-CoV-2 nucleocapsid viral antigens from direct anterior nasal swab specimens without transport media from symptomatic individuals who are suspected of COVID-19 by their healthcare provider within the first six days of symptom onset. The EUA is limited to laboratories certified under CLIA to perform moderate or high-complexity testing. For more information on this topic, download the full white paper.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of RF Globalnet? Subscribe today.
